
#Atomic table pdf
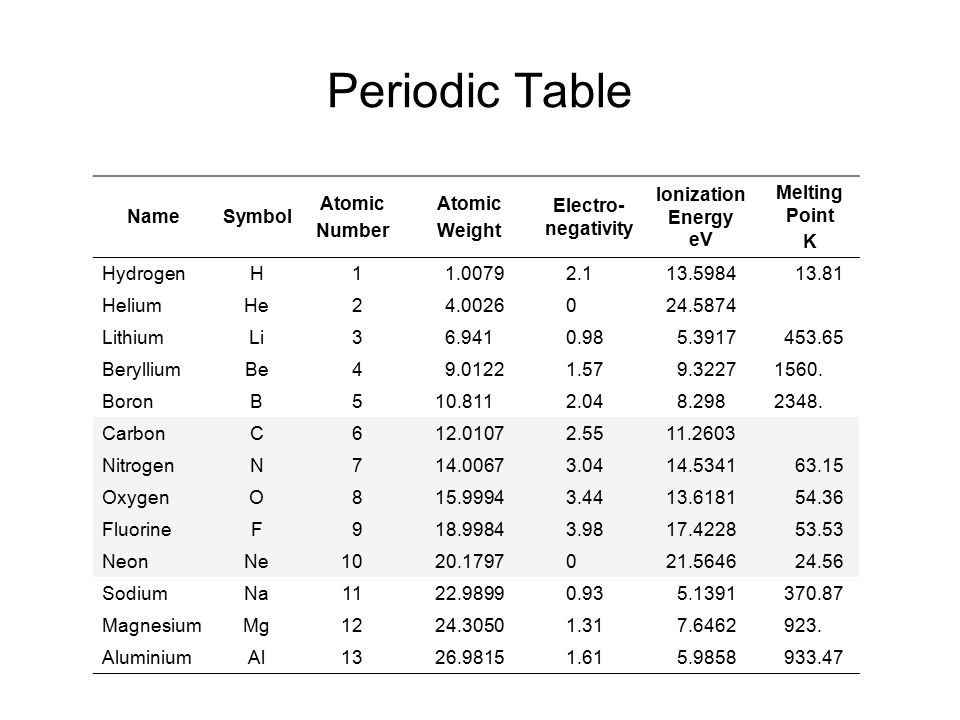
Now available: history of the periodic tableĬhoose elements by name, by atomic number, by symbol, by massĬlick here for the history of the periodic table.Ĭlick here to download a PDF version from that periodic table An interactive, printable extended version of the Periodic table of chemical elements of Mendeleev (who invented the periodic table). According to the data, it is the seventeenth most abundant element found on earth. Carbon is a nonmetal and tetravalent i.e has 4 electrons in the valence shell. The atomic number of carbon is 6 and the atomic mass is 12.01gmol-1. Title: Periodic Table Author: Michael Dayah Created Date: 12:38:25 AM. Carbon is an element represented by C, it belongs to the 14th period in the periodic table.
/PeriodicTableSigFig-NoBG-56a12da75f9b58b7d0bcd00f.png)
Since version 20.10 it is a default database engine (before engineOrdinary was used). Phone: +31 152 610 900 chemical element contains a link to a page that explains its chemical properties, health effects, environmental effects, application data, an image and also information of the history/inventor of each element. Atomic Actinoids (Actinides) Lanthanoids (Lanthanides) Noble gases Other nonmetals Post-transition metals Transition metals Alkaline earth metals Alkali metals Nonmetals. In version 20.5 ClickHouse first introduced database engineAtomic.A chemical element, often simply called an element, is a type of atom which has the same number of protons in its atomic nucleus (i.e., the same atomic number, ( Z ). However, the Periodic table generally displays only the symbol of the element and not its entire name. This is a list of the 118 chemical elements that have been identified as of 2023.



 0 kommentar(er)
0 kommentar(er)
